Research &
Development
Therapeutic focus
TGCT
Tenosynovial Giant Cell Tumour (TGCT) is a typically benign but sometimes aggressively growing tumour in the synovial lining of joints, tendon sheaths or bursae, primarily located in knee, hip, and ankle. TGCT is caused by excessive production of CSF-1 as a result of a chromosome abnormality. TGCT is a serious and chronically debilitating disease in which patients experience severe pain, swelling, loss of function of the affected joints and diminished quality of life. TGCT is usually found in adults aged 20-50 years old, but it may also be found in children1.
Symptoms of these tumours are often initially like those of other conditions, such as sports injuries or arthritis, so they may initially be difficult to diagnose and treat effectively. TGCT can affect nearly every aspect of a person’s daily life. Common everyday activities, such as walking or shopping, can be very tough for people with TGCT.
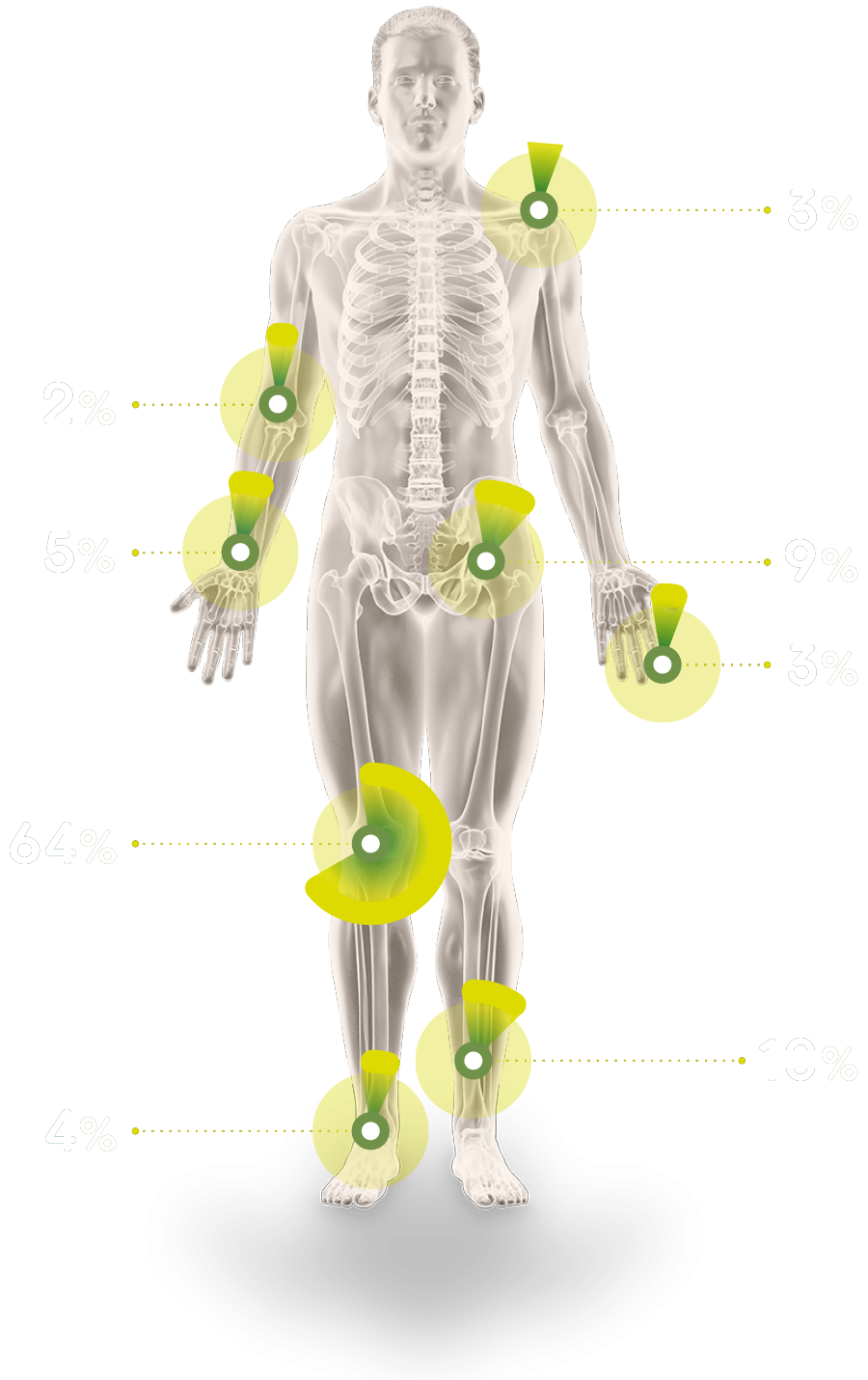
There are generally two types of TGCT: localised TGCT and diffuse TGCT, which is less common and more difficult to treat. The estimated global incidence of TGCT overall is 43 cases per million patient years, with 4 per million patient years of these estimated as being diffuse TGCT2.
Surgery is often the first-line treatment approach. Some diffuse tumours may be inoperable due to the size or location of the tumour within the joint. Surgery may damage portions of the joint lining and high recurrence rates of up to 50%3 for diffuse TGCT, often requiring multiple surgeries, have been reported. Excessive damage to the joint may lead to the requirement of a joint replacement or amputation.
Leveraging the preliminary efficacy, safety and PK-PD data in more than 450 patients, we believe emactuzumab could add significant advantages over existing treatment options for patients with TGCT. TANGENT, our Phase III registrational clinical study, will add to the encouraging data we have generated already, and advance our TGCT programme into the late stages clinical development. We ultimately aim to provide a best-in-class treatment option to address the unmet needs, and improve the quality of life of TGCT patients suffering from this chronically debilitating disease.
Ray Barlow
Chief Executive Officer
Scientific background
CSF-1R inhibition
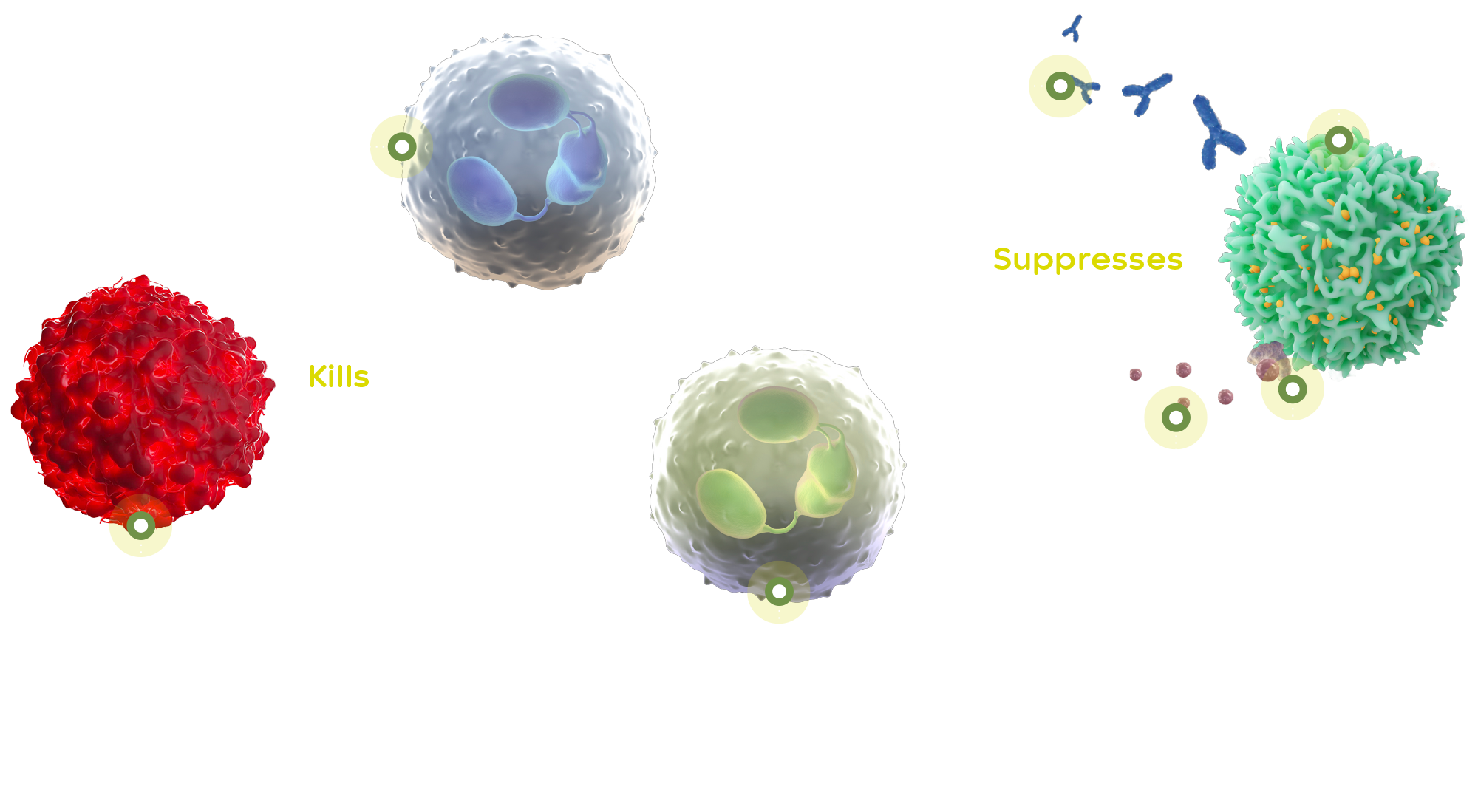
The monoclonal antibody emactuzumab is a potent, specific inhibitor of CSF-1R and data generated to date show its potential as a therapeutic platform targeting serious macrophage-driven inflammatory, fibrotic and neovascular diseases. The CSF-1 receptor, via its binding to two regulatory cytokines, CSF-1 and IL-34, is critically involved in the regulation of macrophages and related cells in multiple biological processes across many organ systems, making it an attractive target with broad therapeutic applications.
TGCT is caused by a translocation error in chromosome 1 and chromosome 2, which results in an overproduction of a protein called colony stimulative factor 1 (CSF-1).
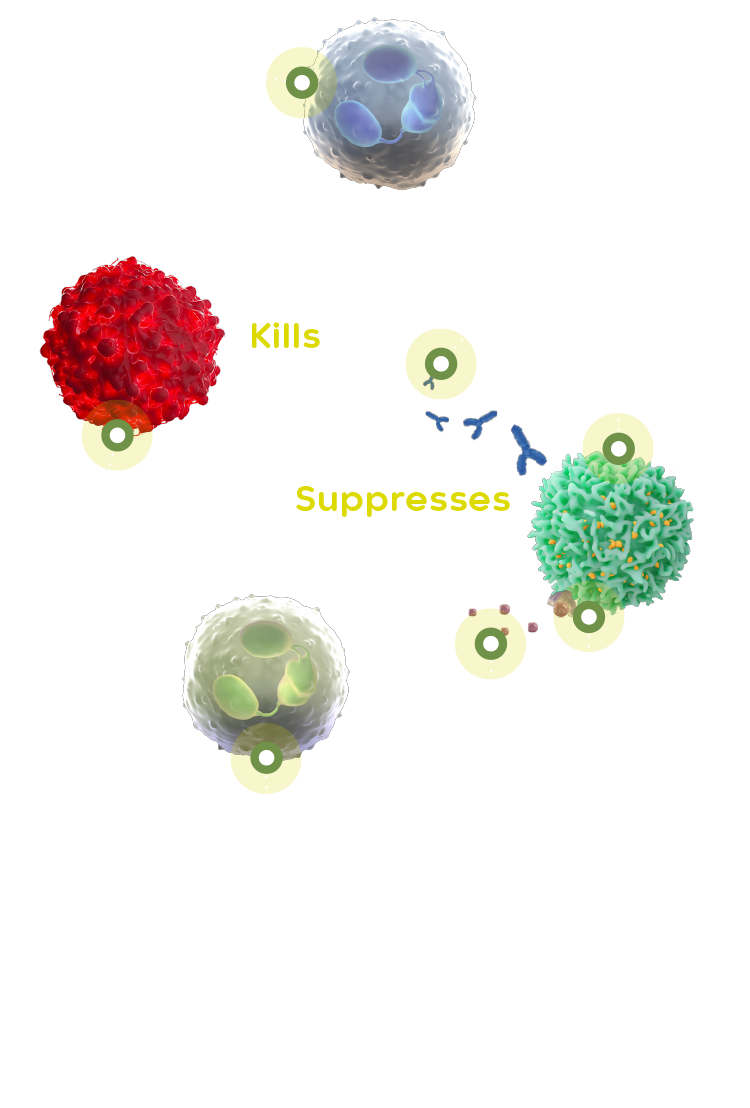
In healthy cells, CSF-1 helps white blood cells to grow and develop. The overproduction of CSF-1 triggers the migration of tumour-associated macrophages (TAMs) to tumour sites. TAMs suppress the T-cell mediated immune response.
Emactuzumab blocks the CSF-1R on TAMs, enhancing T-cell infiltration and anti-tumour T-cell immune response. Emactuzumab was originally discovered and developed by Roche and has been tested in several phase 1a/b studies as monotherapy and in combination with other agents, including chemotherapeutics and immunotherapies in patients with a variety of solid tumours. It was demonstrated that emactuzumab monotherapy led to a substantial tumour response (ORR of 71%)4 and improvements in functional ability of patients with good tolerability and a manageable safety profile in patients with diffuse TGCT.
Tumour Microenvironment